Answer:
The reaction is not at equilibrium and reaction must run in reverse direction.
Step-by-step explanation:
At the given interval, concentration of
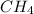 =
=
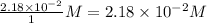
Concentration of
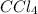 =
=
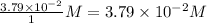
Concentration of
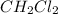 =
=
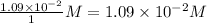
Reaction quotient,
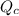 , for this reaction =
, for this reaction =
![([CH_(2)Cl_(2)]^(2))/([CH_(4)][CCl_(4)])](https://img.qammunity.org/2020/formulas/chemistry/college/grmvusfynub6zl98mj3mxnqted6hthtg8y.png)
species inside third bracket represents concentrations at the given interval.
So,
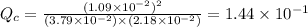
So, the reaction is not at equilibrium.
As,
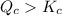 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce
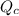 and make it equal to
and make it equal to
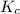 .
.