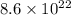Answer:
The total number of electrons is
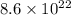
(3) is correct option.
Step-by-step explanation:
Given that,
The current equation is
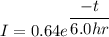
We know that,
The formula of charge
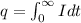
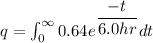
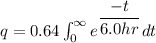
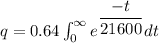
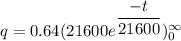
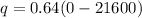
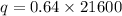
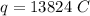
We need to calculate the number of electron
Using formula of charge
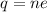
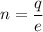
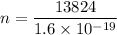
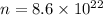
Hence, The total number of electrons is