Answer :
(i) The value of equilibrium constants for this reaction is, 10
(ii) The value of equilibrium constants for this reaction is, 0.1
Explanation :
The given equilibrium reaction is,
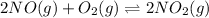
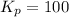
Now we have to determine the equilibrium constants for the following equilibrium reactions.
(i)
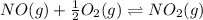
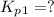
From the given reaction we conclude that, the reaction (i) will takes place when the given main reaction will be multiplied by half (1/2). That means when reaction will be half then the equilibrium constant will be:
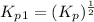
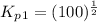
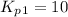
The value of equilibrium constants for this reaction is, 10
(ii)
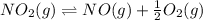
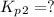
From the given reaction we conclude that, the reaction (ii) will takes place when the reaction (i) will be reverse. That means when reaction will be reverse then the equilibrium constant will be:
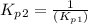
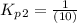
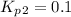
The value of equilibrium constants for this reaction is, 0.1