Answer:
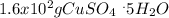
Step-by-step explanation:
Hello,
In this case, the mass of copper (II) sulfate that are available for the 350-g sample is:
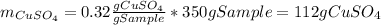
And the mass of water:
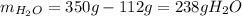
Now, the dissolved copper (II) sulfate at 0°C is:
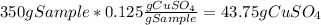
Therefore, the undissolved copper (II) sulfate is:
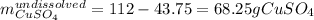
In addition, 238 g of water are equivalent to 13.5 moles of water, nevertheless, as a pentahydrate is obtained, 5 moles of water correspond to:
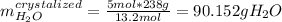
Therefore, the mass of copper (II) sulfate pentahydrate turns out:
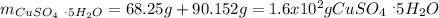
Best regards.