Answer:
The energy level is 5.
Step-by-step explanation:
Given that,
n = 3
l = 2
We know that,
l shows the number of sub-shells and define the number of angular nodes.
n shows the number of electron shell.
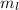 is a quantum number. It is define the number of energy level in a sub-shells .
is a quantum number. It is define the number of energy level in a sub-shells .
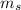 is define the spin of the electron.
is define the spin of the electron.
So, The quantum number is
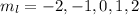
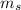 is
is
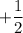 and
and
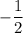 for every energy level.
for every energy level.
The energy level is 5.
Hence, The energy level is 5.