Answer:
Group 12, element is mercury
Step-by-step explanation:
Elements of group 12 are Zn, Cd and Hg.
Valence shell electronic configuration of group 12 elements is:
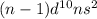
Elements of group 12 elements generally exist in +2 oxidation states in their compounds.
However, Hg also form series of compounds in which oxidation state of Hg is +1.
For example,
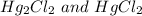
Mercury is a shiny silver colured.
When mercury reacts with sulfur, a odorless red colored ppt is formed.
So among given option, group 12 is the correct answer and element is mercury.