Answer:
Answer for the given statements: (1) T , (2) F , (3) T , (4) T , (5) F
Step-by-step explanation:
At the given interval, concentration of CO =
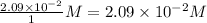
Concentration of Cl_{2} =
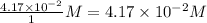
Concentration of COCl_{2} = \frac{0.103}{1}M=0.103M
Reaction quotient,
 , for this reaction =
, for this reaction =
![([COCl_(2)])/([CO][Cl_(2)])](https://img.qammunity.org/2020/formulas/chemistry/college/7zil2vxjvqllmfq6xz1is803l8zvi6aix2.png)
species inside third bracket represents concentrations at the given interval.
So,
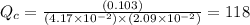
So, the reaction is not at equilibrium.
As Q_{c}> K_{c} therefore reaction must run in reverse direction to reduce Q_{c} and make it equal to K_{c}. That means
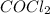 (g) must be consumed and CO must be produced.
(g) must be consumed and CO must be produced.