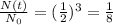Answer:
3
Step-by-step explanation:
The half-life is the time it takes for the amount of radioactive isotope to halve. Therefore, we have:
- After 1 half-life, only 1/2 of the element will be left
- After 2 half-lives, only 1/4 of the element will be left
- After 3 half-lives, only 1/8 of the element will be left
So, it will take 3 half-lives for the element to become 1/8 of its original amount.
Mathematically, this can be also verified by using the equation
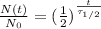
where
N(t) is the amount of the element left at time t
N0 is the initial amount of the element
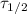 is the half-life
is the half-life
Substituting
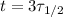 (3 half-lives), we find
(3 half-lives), we find