Answer:
1.479 A
Step-by-step explanation:
Expression for de Broglie wave length
λ =
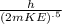
Where m is mass of neutron ,h is plank constant and KE is kinetic energy of
proton.
Kinetic Energy ( KE) = 3 / 2 kT
=1.5 X 1.38 X 10⁻²³ X 290 = 600.3 X 10⁻²³ J
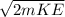 =
=
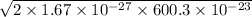
44.777 x 10⁻²⁵
λ =
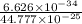
=1.479 A.