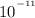Answer: 4.32 X
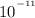
Explanation: Given concentration of hydroxide ions = 2.31 x
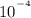
As we know that Kw ( ionic product of water) at 25 °C =
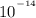
and Kw is the product of concentration of hydronium ions and hydroxide ions.
Thus,
Kw =
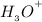
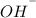
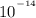 =
=
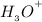 X 2.31 x
X 2.31 x
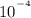
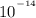 / 2.31 x
/ 2.31 x
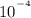 =
=
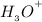
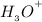 = 4.32 X
= 4.32 X
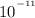
Thus, the concentration of hydronium ions in a solution = 4.32 X