Answer:
Temperature, T = 3.62 kelvin
Step-by-step explanation:
It is given that,
Total number of gas molecules,
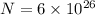
Her body is converting chemical energy into thermal energy at a rate of 125 W, P = 125 W
Time taken, t = 6 min = 360 s
Energy of a gas molecules is given by :
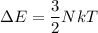
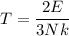 , k is Boltzmann constant
, k is Boltzmann constant
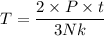
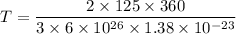
T = 3.62 K
So, the temperature increases by 3.62 kelvin. Hence, this is the required solution.