Answer:
(a) 0.3778 eV
(b) Ratio = 0.0278
Step-by-step explanation:
The Bohr's formula for the calculation of the energy of the electron in nth orbit is:
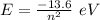
(a) The energy of the electron in n= 6 excited state is:
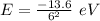
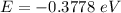
Ionisation energy is the amount of this energy required to remove the electron. Thus, |E| = 0.3778 eV
(b) For first orbit energy is:
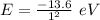
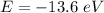
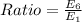
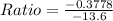
Ratio = 0.0278