Answer:
Option A as well as Option D are correct statements.
Step-by-step explanation:
Each member of the group has the identical electron configuration in the outer shell For example VIIA or 17th group of the periodic table contain Halogens that are Fluorine, Chlorine, Bromine, Iodine and Astatine.
All these elements have same number of valence electrons in its outer shell that is 7.
For example F-9
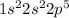
Cl-17
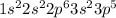
Hence the general electronic configuration of halogens for the Valence shell is
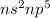
Because of this reason the elements in a group have similar Chemical properties since electrons play a role in the chemical property of an element.