Answer:
Option A) n
Step-by-step explanation:
In accordance to Quantum Mechanical model of an atom:
- The Principle Quantum number (n) gives the description of the shell of an electron and the energy level of an electron in an atom.
- The angular momentum also referred to as Azimuthal Quantum number (l) gives the description of the shape of the orbitals and helps in determination of angular momentum magnitude.
- The magnetic quantum number (
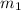 ) describes the energy levels or the number of orbitals contained in a subshell and the way these are oriented within.
) describes the energy levels or the number of orbitals contained in a subshell and the way these are oriented within.
- The spin quantum no. (
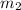 ) determines the elelctron spin's direction which may be (
) determines the elelctron spin's direction which may be (
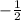 ) or (
) or (
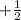 ).
).