Answer:
minimum uncertainty is 2.9 nm
Step-by-step explanation:
Given data
electron's speed = 2.0 x 10^6 m/s
uncertainty = 10%
to find out
minimum uncertainty
solution
first we find out here electron momentum that is mass × velocity
and we know mass is 9.10 ×
 kilograms
kilograms
so momentum p = 9.10 ×
 × 2.0 x
× 2.0 x
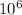
momentum p = 1.82 ×
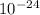 kilograms m /s
kilograms m /s
and uncertainty = 10% so
change in momentum Δp = 1.82 ×
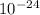 × 10%
× 10%
so Δp = 1.82 ×
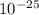 kilograms m /s
kilograms m /s
and
minimum uncertainty Δx will be calculated by heisenberg uncertainty principle that is
ΔpΔx ≥ h / 4π
so Δx = h / 4π Δp
Δx = 6.625×
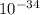 / 4(π) 1.82 ×
/ 4(π) 1.82 ×
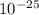
Δx = 2.9 ×
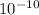 m
m
so minimum uncertainty is 2.9 nm