Answer:
70.872 mL
Step-by-step explanation:
The reaction of Sodium borohydrate with sulfuric acid to form diborane is shown below as:
2 NaBH₄ + 2 H₂SO₄ = B₂H₆ + 3 H₂ + 2 NaSO₄
Given mass of B₂H₆ = 0.579 g
Molar mass of B₂H₆ = 27.66 g/mol
The formula for the calculation of moles is shown below:

Thus, moles of B₂H₆are:
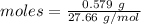
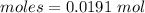
From the reaction,
1 mole of B₂H₆ is produced when 2 moles of NaBH₄ react with acid.
So,
0.0191 moles of B₂H₆ is produced when 2×0.0191 moles of NaBH₄ react with acid.
Moles of NaBH₄ required = 0.0382 moles
Given, Molarity of NaBH₄ = 0.539 M
Molarity = Moles / Volume
Volume = Moles / Molarity = 0.0382 moles / 0.539 M = 0.070872 L
Also, 1L = 1000 mL
So,
Volume = 70.872 mL