Answer:
time taken is 2.49 x
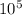 year
year
Step-by-step explanation:
Given data
energy = 3.27 MeV
mass = 1 kg
to find out
time period
solution
we know that deuterium atomic mass unit = 1.66053886 x
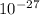 kg
kg
and we know that energy release = 1 kg × 3.27 / 2×2×1.66053886 x
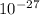
energy release = 4.9231 x
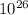 MeV
MeV
energy release = 4.9231 x
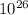 × 1.6 x
× 1.6 x
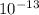 = 7.87 x
= 7.87 x
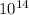 J
J
so at 100 W
energy = power × time
time = 7.87 x
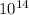 / 100
/ 100
time = 7.87 x
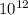 s = 7.87 x
s = 7.87 x
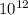 / 3.15 x
/ 3.15 x
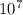 = 2.49 x
= 2.49 x
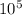 year
year
so time taken is 2.49 x
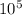 year
year