Answer:
Answer is given below.
Step-by-step explanation:
Anode is that electrode where oxidation occurs. Cathode is that electrode where reduction occurs.
In cell representation, half cell present left to salt-bridge notation
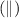 is anodic system and another half cell present right to salt-bridge notation
is anodic system and another half cell present right to salt-bridge notation
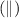 is cathodic system.
is cathodic system.
So anode is Cu and cathode is Ag.
oxidation:
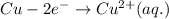
[reduction:
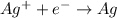 ]
]
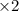
-----------------------------------------------------------------------------------------------
chemical equation:
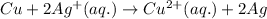
Oxidizing agent is that species which takes electron from another species. Here
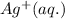 takes electron from Cu. Hence
takes electron from Cu. Hence
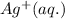 is the oxidizing agent.
is the oxidizing agent.
Reducing agent is that species which gives electron to another species. Here Cu gives electron to
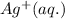 . Hence Cu is the reducing agent.
. Hence Cu is the reducing agent.