Answer:
The energy in electron volts of a single photon of these X‑rays is 46697.5 eV.
Step-by-step explanation:
Given that,
Wavelength = 0.0265 nm
We need to calculate the energy
Using formula of energy
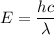
Where, h = plank constant
c = speed of light
 = wavelength
= wavelength
Put the value into the formula
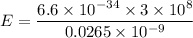
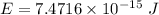
We know that,
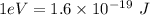
The energy in eV
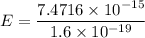
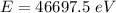
Hence, The energy in electron volts of a single photon of these X‑rays is 46697.5 eV.