The question is incomplete, here is the complete question:
A chemist must prepare 550.0 mL of hydrochloric acid solution with a pH of 1.60 at
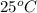 . He will do this in three steps: Fill a 550.0 mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.
. He will do this in three steps: Fill a 550.0 mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.
Answer: The volume of concentrated hydrochloric acid solution is 1.7 mL.
Step-by-step explanation:
To calculate the hydrogen ion concentration, we use the formula:
![pH=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/d4u8c7rky5aqengst85apsbxbk128yl4er.png)
We are given:
pH = 1.60
Putting values in above equation, we get:
![1.60=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/32zitm0hhxzurjnrjnbrp9th0r85mnufxs.png)
![[H^+]=antilog (-1.60)](https://img.qammunity.org/2022/formulas/chemistry/college/ezglo4i97pgjnlff1sjklz36ipwqgtqcc9.png)
![[H^+]=0.0251M](https://img.qammunity.org/2022/formulas/chemistry/college/dpo502eu5qui3xlw9x2xyuafjty97v0599.png)
To calculate the volume of concentrated solution, we use the equation:
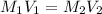
where,
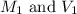 = molarity and volume of concentrated acid solution
= molarity and volume of concentrated acid solution
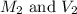 = molarity and volume of diluted acid solution
= molarity and volume of diluted acid solution
We are given:
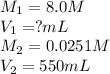
Putting values in above equation, we get:
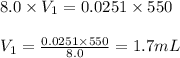
Hence, the volume of concentrated hydrochloric acid solution is 1.7 mL.