Answer:
KOH is a strong base,
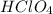 is a strong acid,
is a strong acid,
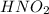 is a weak acid, NaBr is a soluble salt and
is a weak acid, NaBr is a soluble salt and
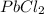 is an insoluble salt
is an insoluble salt
Step-by-step explanation:
According to solubility rule, all chlorides, bromides and iodides of metal ions are soluble except for
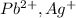 and Hg^{+}[/tex].
and Hg^{+}[/tex].
Therefore NaBr is a soluble salt and
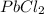 is an insoluble salt.
is an insoluble salt.
There are seven strong acids : HCl, HBr, HI,
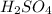 ,
,
 ,
,
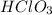 and
and
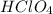 .
.
All group IA metal hydroxides e.g. NaOH, KOH etc. are strong base. Also,
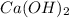 ,
,
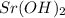 and
and
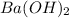 are strong bases.
are strong bases.
So, KOH is a strong base,
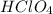 is a strong acid and
is a strong acid and
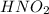 is a weak acid.
is a weak acid.