Answer: The mass defect for the formation of phosphorus-31 is 0.27399
Step-by-step explanation:
Mass defect is defined as the difference in the mass of an isotope and its mass number.
The equation used to calculate mass defect follows:
![\Delta m=[(n_p* m_p)+(n_n* m_n)]-M](https://img.qammunity.org/2020/formulas/chemistry/college/5bdxz8rq0j0u327tau9vud72y9upvsk2mm.png)
where,
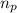 = number of protons
= number of protons
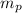 = mass of one proton
= mass of one proton
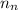 = number of neutrons
= number of neutrons
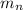 = mass of one neutron
= mass of one neutron
M = mass number of element
We are given:
An isotope of phosphorus which is
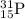
Number of protons = atomic number = 15
Number of neutrons = Mass number - atomic number = 31 - 15 = 16
Mass of proton = 1.00728 amu
Mass of neutron = 1.00866 amu
Mass number of phosphorus = 30.973765 amu
Putting values in above equation, we get:
![\Delta m=[(15* 1.00728)+(16* 1.00866)]-30.973765\\\\\Delta m=0.27399](https://img.qammunity.org/2020/formulas/chemistry/college/ckodzemxdcmd4s1mhv7ljcqa4u3qlhom2l.png)
Hence, the mass defect for the formation of phosphorus-31 is 0.27399