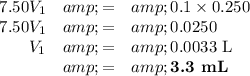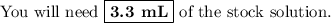Answer:
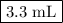
Step-by-step explanation:
You must convert 30 % (m/v) to a molar concentration.
Assume 1 L of solution.
1. Mass of NaOH
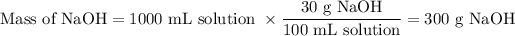
2. Moles of NaOH
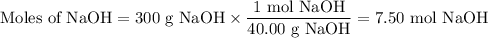
3. Molar concentration of NaOH
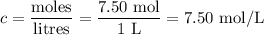
4. Volume of NaOH
Now that you know the concentration, you can use the dilution formula .
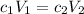
to calculate the volume of stock solution.
Data:
c₁ = 7.50 mol·L⁻¹; V₁ = ?
c₂ = 0.1 mol·L⁻¹; V₂ = 250 mL
Calculations:
(a) Convert millilitres to litres
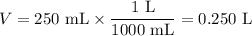
(b) Calculate the volume of dilute solution