Answer: The correct answer is etching.
Step-by-step explanation:
Etching is defined as the process in which a strong acid is used to cut in the parts of unprotected parts of a metal surface to create a design.
When buffered hydrofluoric acid is treated with glass containing silicon dioxide
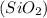 , it leads to the formation of silicon tetrafluoride and water.
, it leads to the formation of silicon tetrafluoride and water.
When silicon tetrafluoride is further treated with hydrofluoric acid, it leads to the formation of hydrofluorosilicic acid.
The chemical equations for the above process is:
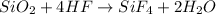
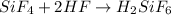
Thus, the correct answer is etching.