Answer : The molecular diameter is,
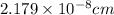 [/tex]
[/tex]
Explanation :
Formula used for mean free path :
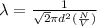
where,
 = Mean free path =
= Mean free path =
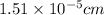
d = diameter of molecule = ?
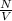 = number of molecules per unit volume =
= number of molecules per unit volume =
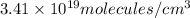
Now put all the given values in this formula, we get:
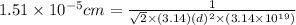
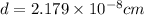 [/tex]
[/tex]
Therefore, the molecular diameter is,
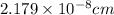 [/tex]
[/tex]