Answer: The elevation in boiling point is 1.024°C.
Step-by-step explanation:
To calculate the elevation in boiling point, we use the equation:
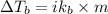
where,
i = Van't Hoff factor = 2 (for NaCl)
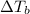 = change in boiling point = ?
= change in boiling point = ?
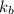 = boiling point constant =
= boiling point constant =
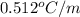
m = molality = 1.0 m
Putting values in above equation, we get:
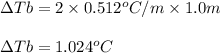
Hence, the elevation in boiling point is 1.024°C.