Answer : The mass percentage of arsenic in the pesticide is, 5.22 %
Explanation :
First we have to calculate the moles of
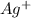 .
.
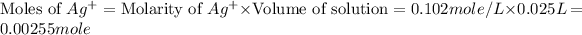
Now we have to calculate the moles of arsenic.
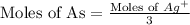
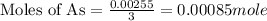
Now we have to calculate the mass of arsenic.
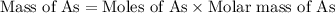
Molar mass of arsenic = 74.9 g/mole
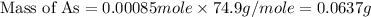
Now we have to calculate the mass percentage of arsenic in the pesticide.
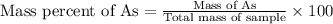
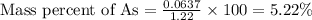
Therefore, the mass percentage of arsenic in the pesticide is, 5.22 %