Answer:
4276.98 years
Step-by-step explanation:
t = age of the sample in numbers of years
T = half life of the carbon-14 isotope = 5730 yrs
λ = decay constant of carbon-14
decay constant is given as
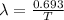
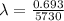
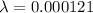
A₀ = activity of Carbon-14 in living plants
A = activity of Carbon-14 after time "t" = (0.596) A₀
Using the equation
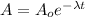
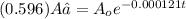
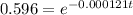
t = 4276.98 years