Answer:
D linear
Step-by-step explanation:
Valence electrons of carbon = 4
Valence electrons of sulfur = 6
The total number of the valence electrons = 4 + 2(6) = 16
The Lewis structure is drawn in such a way that the octet of each atom in the molecule is complete. So,
The Lewis structure is:
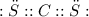
There is no lone pair involved, so, It is of type AB₂.
According to the theory, the atoms will form a geometry in such a way that there is minimum repulsion and maximum stability.
So, it is of linear shape.