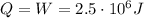Answer:
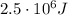
Step-by-step explanation:
According to the 1st law of thermodynamics, we have:
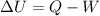
where
 is the change in internal energy of the system
is the change in internal energy of the system
Q is the heat absorbed by the system
W is the work done by the system
We also know the change in internal energy of a system only depends on the change in temperature:
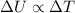
Since here the temperature of the air remains constant, the change in internal energy is zero:
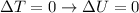
So the first equation becomes
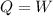
The work done by the system here is
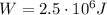
Therefore, the heat added to the system is