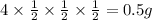A sample of 4 g of cobalt isotope is produced. If the half-life of is 30 years, what will be the mass of the cobalt remaining after 90 years is 0.5 g.
Step-by-step explanation:
After 30 years the amount of cobalt isotope will be half of the original value, which is 2 g. After 60 years means, this will be decremented half of that 2 g which is 1 g. After 90 years, it will be half of the previous value that will be equal to 0.5 g.
(i.e)