Step-by-step explanation:
Formula to calculate how many particles are left is as follows.
N =
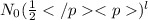
where,
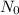 = number of initial particles
= number of initial particles
l = number of half lives
As it is given that number of initial particles is
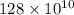 and number of half-lives is 3.
and number of half-lives is 3.
Hence, putting the given values into the above equation as follows.
N =
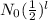
=
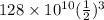
=
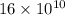
or,
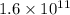
Thus, we can conclude that
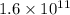 particles of radioactive nuclei remain in the given sample.
particles of radioactive nuclei remain in the given sample.
In five hours we've gone through 5 half lives so the answer is:
particles