Answer:
50%
Step-by-step explanation:
Let assume that there are:
"a" moles of CH4 & "b" moles of C2H2
Then by applying the ideal gas equation:
PV = nRT
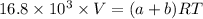
Make (a+b) the subject of the formula:
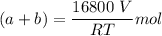 --- (1)
--- (1)
Since 1 mole of CH₄ yields 1 mol of CO₂ & 1 mol of C₂H₂ yields 2 moles of CO₂
Then;
the total moles of CO₂ = (a +2b)
Now:
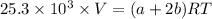
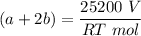 ---- (2)
---- (2)
∴
By solving the above equations
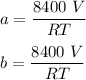
Hence, the estimate of the percentage of methane in the original mixture is:
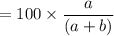
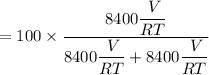
= 50%