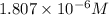Answer : The concentration of unknown
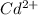 will be,
will be,
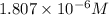
Solution :
The balanced cell reaction will be,
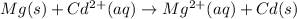
Here magnesium (Mg) undergoes oxidation by loss of electrons, thus act as anode. Cadmium (Cd) undergoes reduction by gain of electrons and thus act as cathode.
Now we have to calculate the concentration of unknown
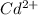 .
.
Using Nernest equation :
![E_(cell)=E^o_(cell)-(0.0592)/(n)\log ([Mg^(2+)])/([Cd^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/yd8r16ucnmrtfyq2egaega5elpa3sik8rl.png)
where,
n = number of electrons in oxidation-reduction reaction = 2
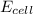 = emf of the cell = 1.80 V
= emf of the cell = 1.80 V
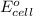 = standard cell potential = 1.97 V
= standard cell potential = 1.97 V
![[Mg^(2+)]](https://img.qammunity.org/2020/formulas/chemistry/college/cv6hvdw1ngs1847bo8u9v3zixi1zt8zm1k.png) = concentration of magnesium ion = 1.00 M
= concentration of magnesium ion = 1.00 M
![[Cd^(2+)]](https://img.qammunity.org/2020/formulas/chemistry/college/gtislri2oskw56gn9ojoryikvkubv3kn7p.png) = concentration of cadmium ion = ?
= concentration of cadmium ion = ?
Now put all the given values in the above equation, we get
concentration of unknown
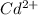 .
.
![1.80=1.97-(0.0592)/(2)\log ((1.00))/([Cd^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/ch6rm8hxdzewfysn7mgo06vpieej7djnpm.png)
![[Cd^(2+)]=1.807* 10^(-6)M](https://img.qammunity.org/2020/formulas/chemistry/college/ksrya5gpw3ot6uwtkbftzgccovx0g3bxv7.png)
Therefore, the concentration of unknown
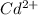 will be,
will be,