Answer:
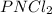
Step-by-step explanation:
Hello!
In this case, when determining empirical formulas by knowing the by-mass percent, we first must assume the percentages as masses so we can compute the moles of each element:
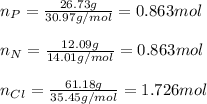
Now, for the determination of the subscript of each element in the empirical formula, we divide the moles by the fewest moles (P or N):
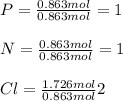
Thus, the empirical formula is:
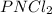
Regards!