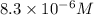Answer:
Concentration of hydroxide-ion at equivalence point =
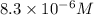
Step-by-step explanation:
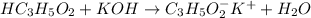
1 mol of
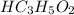 reacts with 1 mol of KOH to produce 1 mol of
reacts with 1 mol of KOH to produce 1 mol of
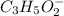
At equivalence point, all
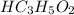 gets converted to
gets converted to
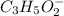 .
.
Moles of
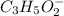 produced at equivalence point is equal to moles of KOH added to reach equivalence point.
produced at equivalence point is equal to moles of KOH added to reach equivalence point.
So, moles of
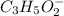 produced =
produced =
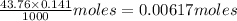
Total volume of solution at equivalence point = (25.00+43.76) mL = 68.76 mL
Concentration of
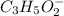 at equivalence point =
at equivalence point =
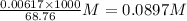
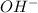 produced at equivalence point is due to hydrolysis of
produced at equivalence point is due to hydrolysis of
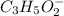 . We have to construct an ICE table to calculate concentration of
. We have to construct an ICE table to calculate concentration of
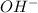 at equivalence point.
at equivalence point.
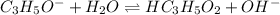
I:0.0897 0 0
C: -x +x +x
E: 0.0897-x x x
![([HC_(3)H_(5)O_(2)][OH^(-)])/([C_(3)H_(5)O_(2)^(-)])=K_(b)(C_(3)H_(5)O_(2)^(-))=(10^(-14))/(K_(a)(HC_(3)H_(5)O_(2)))](https://img.qammunity.org/2020/formulas/chemistry/college/9xgi8ghkk7f5iis2k9t5155dicas44vyyd.png)
species inside third bracket represent equilibrium concentrations
So,
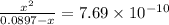
or,
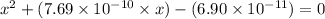
So,
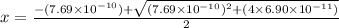 M =
M =
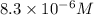
So, concentration of hydroxide-ion at equivalence point = x M =