Answer: the time it takes for one-half of the sample to decay.
Step-by-step explanation:
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
Radioactive decay process is a type of process in which a less stable nuclei decomposes to a stable nuclei by releasing some radiations or particles like alpha, beta particles or gamma-radiations. The radioactive decay follows first order kinetics.
Half life is represented by
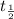
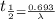
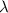 = rate constant
= rate constant
Thus the half-life of a radioisotope is the time it takes for one-half of the sample to decay.