Step-by-step explanation:
(i) The equilibrium reaction equation will be as follows.
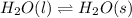
A reaction in which there will be absorption of heat energy is known as endothermic reaction.
A reaction in which there will be release of heat energy is known as exothermic reaction.
As liquid state of water is changing into solid state. So, it means that molecules of water came close to each other. Hence, there will be release of heat this means that reaction is exothermic in nature.
Hence, phase change from liquid to solid will be exothermic in nature.
Latent heat of fusion is defined as the amount of energy necessary to convert 1 gram of a solid into liquid state at its melting point.
So, when solid state of water changes into liquid state then it means energy is absorbed by the molecules of ice due to which they have gained kinetic energy. Hence, they moved away from each other leading to formation of liquid state of water.
Latent heat of freezing of liquid water is 334 J/g.
Specific heat of liquid water is 4.186
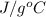
Specific heat of steam is 1.996
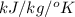
Specific heat of ice is 2.1
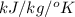
(ii) The equilibrium reaction equation will be as follows.
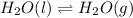
As liquid state of water is changing into gaseous or vapor state. So, it means molecules of liquid water has gained kinetic energy hence, they colloid more rapidly with each other.
As a result, heat will be absorbed by the liquid state of water. Hence, heat will be absorbed. Therefore, phase change from liquid to gas will be endothermic in nature.
Whereas when gaseous state of water will change into liquid state then heat will be released during this process of condensation. As a result, in that case reaction will be exothermic in nature.