Answer: a.
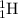
b. Atomic number of the nucleus formed is 89 which is actinium.
Step-by-step explanation:
Nuclear fission reactions are defined as the reactions in which a heavier nuclei breaks down in two or more smaller nuclei.
In a nuclear reaction, the total mass and total atomic number remains the same.
For the given fission reaction:
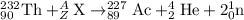
To calculate A, mass number
Total mass on reactant side = total mass on product side
232 + A = 227+4+2
A = 1
To calculate Z, atomic number
Total atomic number on reactant side = total atomic number on product side
90 + Z = 89 +2+ 0
Z = 1
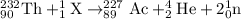
The isotopic symbol of element with atomin number of 1 and mass number of 1 is
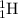
The atomic number of the nucleus formed is 89 which is actinium.