Answer:
520 mg Al(OH)₃
Step-by-step explanation:
The reaction that takes place is:
- Al(NO₃)₂ + 3KOH → Al(OH)₃ + 3KNO₃
Now we calculate how many moles of each reagent were added, using their concentrations and volumes:
- Al(NO₃)₂ ⇒ 50.0 mL * 0.200 M = 10 mmol Al(NO₃)₂
- KOH ⇒ 200.0 mL * 0.100 M = 20 mmol KOH
10 mmol of Al(NO₃)₂ would react completely with (10*3) 30 mmol of KOH, there are not as many KOH mmol, so KOH is the limiting reactant.
We calculate how many moles of Al(OH)₃ are produced, using the limiting reactant:
- 20 mmol KOH *
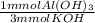 = 6.67 mmol Al(OH)₃
= 6.67 mmol Al(OH)₃
Finally we convert mmol of Al(OH)₃ to mg, using its molar mass:
- 6.67 mmol Al(OH)₃ * 78 mg/mmol = 520 mg Al(OH)₃