The given question is incomplete. the complete question is : If 5.15 g
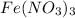 is dissolved in enough water to make exactly 323 ml of solution, what is the molar concentartion of nitrate ion.
is dissolved in enough water to make exactly 323 ml of solution, what is the molar concentartion of nitrate ion.
Answer: The molar concentartion of nitrate ion is 0.195.
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
Given : 5.15 g of
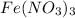 is dissolved
is dissolved
Volume of solution = 323 ml
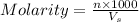
where,
n= moles of solute
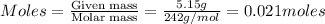
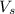 = volume of solution = 323 ml
= volume of solution = 323 ml
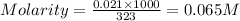
As 1 M of
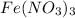 gives 3 M of
gives 3 M of
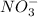 ions.
ions.
Thus 0.065 M of
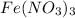 gives =
gives =
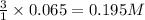 of
of
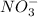 ions.
ions.
The molar concentartion of nitrate ion is 0.195.