Answer:
Step-by-step explanation:
The density of a gas can be obtained using the gas ideal equation and the molar mass of the gas.
This is the decution of the final formula:
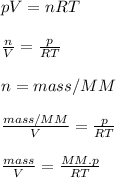
Now, you just need to substitute values:
- R = 0.08206 atm-liter / k-mol
- d = 32.0 g/mol × 0.9869 atm / [0.08206 atm-liter/k-mol × 273.15K]
- d = 1.4 g/liter (using two significant figures)
As you see, I have not used the 4.8 grams datum. That is because the density of the gases may be calculated from the temperature, pressure and molar mass of the gas, using the ideal gas equation.
Since, you have the mass of gas, you might use this other procedure:
- Volume of 1 mol of gas at STP: about 22.4 liter/mol
- Mass of 1 mol of oxygen gas: 32.0 g/mol (the molar mass)
- number of moles in 4.8 g of oxygen = 4.8 g / 32.0 g/mol = 0.15 mol
- Volume of 0.15 mol of oxygen: 0.15 mol × 22.4 liter/mol = 3.36 liter
- Density = mass / volume = 4.8 g / 3.36 liter = 1.4 g/liter (same result)