Answer:
Choice A: atom X (20 nucleons, 10 out of which are protons) and atom Y (21 neutrons, 10 out of which are protons) are isotopes of each other.
Step-by-step explanation:
Two atoms are isotopes if they contain the same number of protons but different numbers of neutrons.
The table here lists the atomic number and the mass number of each atom.
- The atomic number of an atom is the same as the number of protons in this atom.
- The mass number of an atom is the number of nucleons in this atom. "Nucleon" is an umbrella term that includes protons and neutrons. The mass number of an atom is thus the same as the number of protons plus the number of neutrons in this atom.
Take atom X as an example:
- Number of protons = Atomic Number = 10.
- Number of protons and neutrons = Mass Number = 20.
- Number of neutrons = Mass Number - Atomic Number = 10.
Repeat this process to find the proton and neutron numbers of atom Y and atom Z:
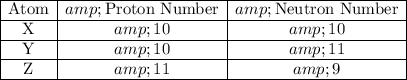 .
.
The atomic number of atom X is the same as that of atom Y. Their atomic number differ from that of atom Z. Their number of neutrons differ. Thus only atom X and atom Y are isotopes of each other.