Answer:
For the Carnot air conditioner working as a heat pump between 63 and 100°F , It would transfer 3.125 Joules of heat for each Joule of electric energy supplied.
Step-by-step explanation:
The process described corresponds to a Carnot Heat Pump. A heat pump is a devices that moves heat from a low temperature source to a relative high temperature destination. To accomplish this it requires to supply external work.
For any heat pump, the coefficient of performance is a relationship between the heat that is moving to the work that is required to spend doing it.
For a Carnot Heat pump, its coefficient of performance is defined as:
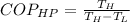 Where:
Where:
- T is the temperature of each heat deposit.
- The subscript H refers to the high temperature sink(in this case the outdoors at 100°F)
- The subscript L refers to the low temperature source (the room at 63°F)
Then, for this Carnot heat pump:
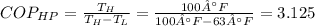
So for each 3.125 Joules of heat to moved is is required to supply 1 Joule of work.