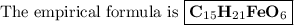Answer:
Step-by-step explanation:
Let's call the unknown compound X.
1. Calculate the mass of each element in 1.23383 g of X.
(a) Mass of C
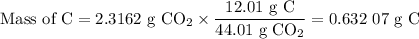
(b) Mass of H
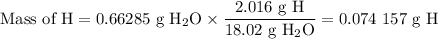
(c)Mass of Fe
(i)In 0.4131g of X
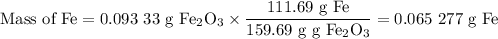
(ii) In 1.2383 g of X
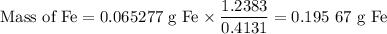
(d)Mass of O
Mass of O = 1.2383 - 0.632 07 - 0.074 157 - 0.195 67 = 0.336 40 g
2. Calculate the moles of each element
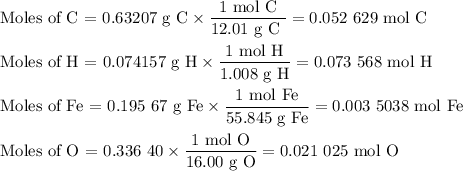
3. Calculate the molar ratios
Divide all moles by the smallest number of moles.
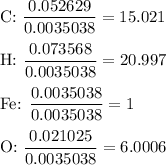
4. Round the ratios to the nearest integer
C:H:O:Fe = 15:21:1:6
5. Write the empirical formula