Step-by-step explanation:
The given data is as follows.
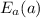 = 27 kJ/mol
= 27 kJ/mol
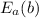 = 35 kJ/mol
= 35 kJ/mol
and,
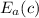 = 15 kJ/mol
= 15 kJ/mol
Activation energy of the overall reaction will be the sum of given activation energies as follows.
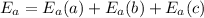
= (27 + 35 + 15) kJ/mol
= 77 kJ/mol
Thus, we can conclude that the overall value of activation energy is 77 kJ/mol.