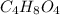Answer:
The molar mass of the organic solid is 120.16 g/mol.
The molecular formula of an organic solid is
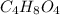
Step-by-step explanation:
Let the molecular mass of an organic solid be
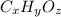
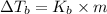
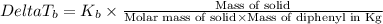
where,
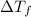 =Elevation in boiling point =
=Elevation in boiling point =
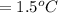
Mass of organic solid= 0.561 g
Mass of diphenyl = 24.9 g = 0.0249 kg (1 kg = 1000 g)
 = boiling point constant = 8.00 °C/m
= boiling point constant = 8.00 °C/m
m = molality
Now put all the given values in this formula, we get
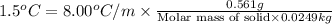
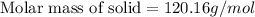
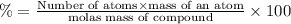
Percentage of carbon in an organic solid = 40.0%
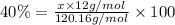
x = 4.0
Percentage of hydrogen in an organic solid = 6.7%
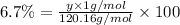
y = 8.0
Percentage of hydrogen in an organic solid = 6.7%

y = 4.0
The molecular formula of an organic solid is