Answer:
Equilibrium will lie to the right.
Step-by-step explanation:
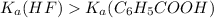
That means HF is stronger acid than
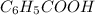 .
.
Alternatively, it can be said that
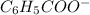 is a stronger base than
is a stronger base than
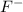 .
.
Therefore HF readily gives proton to
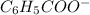 to form
to form
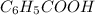 and
and
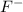 than the reverse reaction.
than the reverse reaction.
So, the equilibrium will lie more towards the right i.e. towards formation of
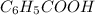 and
and
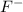 .
.