Answer:
3.078 moles
Step-by-step explanation:
Given:
Number of moles of gas, n = 1.94 moles
Initial temperature, T₁ = 25° C = 298 K
Initial pressure, P₁ = 450 torr
Final temperature, T₂ = 40° C = 313 K
Final pressure, P₂ = 750 torr
now,
we know
PV = nRT
where,
P is the pressure
V is the volume
n is the number of moles
R is the universal gas constant
T is the temperature
now for the initial stage, the above relation comes as:
450 × V = 1.94 × R × 298 .............(1)
for the final stage
750 × V = n × R × 313 ............... (2)
on dividing the equation 2 by 1 we get
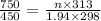
or
n = 3.078 moles