Explanation :
(a)
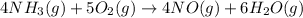
This reaction is combustion reaction in which an oxygen react with a molecule to give its corresponding oxides ans water molecule.
(b)
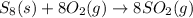
This reaction is a redox reaction or oxidation-reduction reaction in which sulfur get oxidized and oxygen get reduced.
(c)
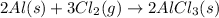
This reaction is a combination reaction in which the two reactants molecule combine to form a large molecule or product.
(d)

This reaction is a decomposition reaction in which a large molecule or reactant decomposes to give two or more molecule or products.
(e)
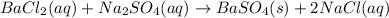
This reaction is a double displacement reaction in which the cation of two reactants molecule exchange their places to give two different products.
(f)
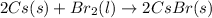
This reaction is a combination reaction in which the two reactants combine to form a large molecule or product.
(g)
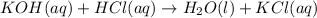
This reaction is a double displacement reaction in which the cation of two reactants molecule exchange their places to give two different products.
(h)
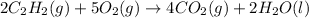
This reaction is combustion reaction in which a hydrocarbon react with an oxygen to give carbon dioxide and water as a products.